
Hypersensitivity Pyrexia, bronchospasm, hyperventilation, rash, and pruritus have been reported in clinical trials with idarucizumab.

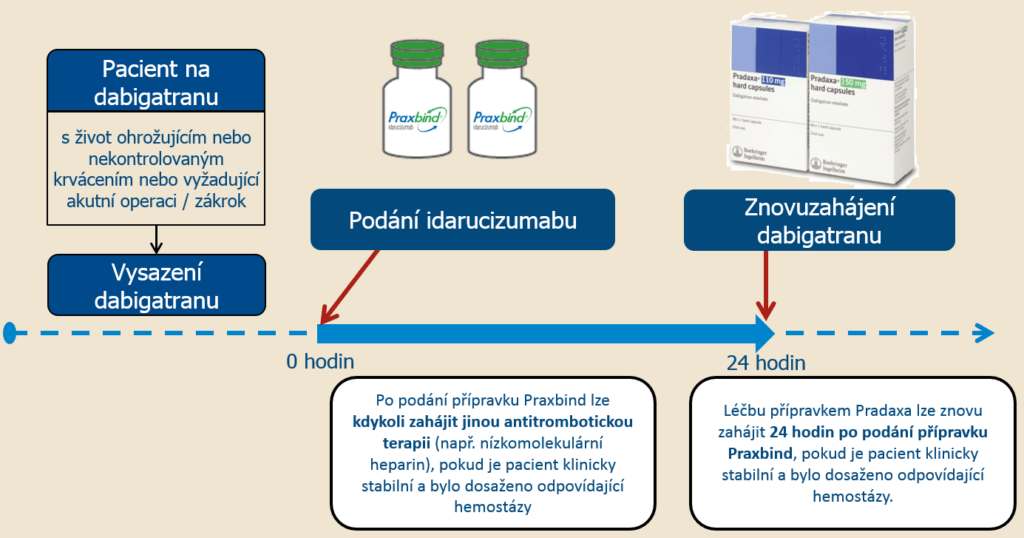
Most of these patients were not on antithrombotic therapy at the time of the event, and in each of these cases, the thrombotic event could be attributed to the underlying medical condition of the patient. Thromboembolic Events In the RE-VERSE AD trial, 33 of 503 patients reported thrombotic events, 11 patients within 5 days after treatment with idarucizumab and 22 patients 6 days or more after treatment with idarucizumab. Of the 503 dabigatran-treated patients in the entire study period, 101 patients died, 19 within the first day after idarucizumab dosing each of these deaths could be attributed either as a complication of the index event or associated with co-morbidities. The adverse reactions reported in ≥5% of patients were constipation (33/503, 7%) and nausea (23/503, 5%). In the RE-VERSE AD™ (RE-VERSal Effects of idarucizumab on Active Dabigatran) trial, a total of 503 dabigatran-treated patients were administered idarucizumab either because they required an emergency surgery or urgent procedure, or because they presented with life-threatening or uncontrolled bleeding. Among those subjects treated with idarucizumab, adverse reactions reported in ≥5% of subjects was headache (12/224, 5%). In these trials, during the treatment period, the overall frequency of adverse events was similar between idarucizumab-treated subjects (55/224, 25%) and placebo-treated subjects (26/105, 25%). In three healthy volunteer clinical trials, 224 subjects were treated with idarucizumab. SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied 16.2 Storage and Handling 17 PATIENT COUNSELING INFORMATION * Sections or subsections omitted from the full prescribing information are not listed.īecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. Intolerance due to Sorbitol Excipient 6 ADVERSE REACTIONS 6.1 Clinical Trials Experience 6.2 Immunogenicity 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy 8.2 Lactation 8.4 Pediatric Use 8.5 Geriatric Use 11 DESCRIPTION 12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action 12.2 Pharmacodynamics 12.3 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility 14 CLINICAL STUDIES 16 HOW Of Serious Adverse Reactions in Patients with Hereditary Fructose
Of Coagulation Parameters 5.3 Hypersensitivity
FULL PRESCRIBING INFORMATION: CONTENTS * 1 INDICATIONS AND USAGE 2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dose 2.2 Preparation 2.3 Administration 2.4 RestartingĪntithrombotic Therapy 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5.1 Thromboembolic


 0 kommentar(er)
0 kommentar(er)
